Renovos Biologics receives FDA Breakthrough Device Designation for its synthetic nanoclay bone fusion gel, RENOVITE®
- RENOVITE® BMP-2 is being developed as an alternative to bone graft materials for interbody spinal fusion
- It is the first product based on RENOVITE®, Renovos’ novel nanoclay therapeutic delivery platform for precision regenerative medicine
Southampton, UK, 4 January 2024 – Renovos Biologics (Renovos) is pleased to announce that it has been granted Breakthrough Device Designation by the US Food and Drug Administration (FDA) for its lead product, RENOVITE® BMP-2 (Bone Morphogenic Protein 2).
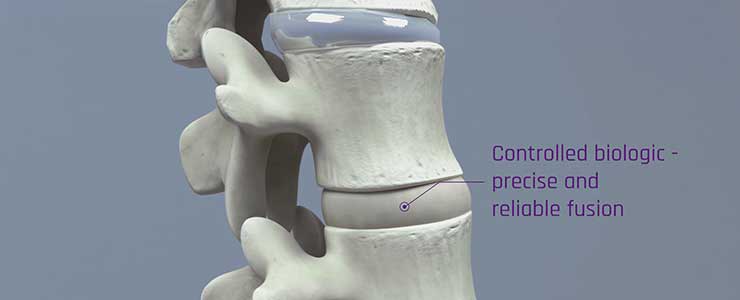
RENOVITE® BMP-2, based on a proprietary synthetic nanoclay gel, is in development as a safer and more effective alternative to currently available bone graft materials. The easy-to-use, injectable gel allows precise, localised bone formation at the target site. It contains BMP-2, a growth factor which promotes in-growth of bone forming cells. The nanoclay gel enables safe, highly-targeted bone fusion, as it does not leach BMP-2, with the gel biodegrading as new bone forms.
Dr Agnieszka Janeczek, Chief Executive Officer of Renovos Biologics, said:
“We are very pleased with the Breakthrough Device Designation from the FDA, being among just one hundred or so companies granted this designation in orthopaedics since the programme’s launch in 2015. This designation is a major milestone in our development journey. The accelerated regulatory feedback and prioritised review will shorten the time to market and allow faster access to expanded treatment options for patients suffering from degenerative disc disease.”
“We are very pleased with the Breakthrough Device Designation from the FDA, being among just one hundred or so companies granted this designation in orthopaedics since the programme’s launch in 2015. This designation is a major milestone in our development journey. The accelerated regulatory feedback and prioritised review will shorten the time to market and allow faster access to expanded treatment options for patients suffering from degenerative disc disease.”
Michael Harris, Chief Executive Officer of Biocomposites, an international medical devices company that engineers, manufactures and markets world leading products for regenerating bone and managing infection in bone and soft tissue, and Renovos’ investor, said: “The granting of FDA Breakthrough Device Designation is a transformational step for Renovos, supporting its goal to rapidly develop and bring to market RENOVITE® – a next-generation drug carrier. At Biocomposites, our expertise in this field ideally positions us to support and enhance RENOVITE®’s progress towards pre-market approval.
The FDA’s Breakthrough Device Designation program is intended to help provide patients more timely access to medical devices which have the potential to provide more effective diagnosis or treatment of life-threatening or irreversibly debilitating conditions, by speeding up the development, assessment and review process. Breakthrough designation is only granted after preliminary evidence has been provided demonstrating a reasonable expectation that the device will provide significant advantages over standard of care. The designation will allow Renovos to have more frequent interaction with the FDA’s regulatory experts when preparing its submissions, followed by prioritised reviews.
Professor Richard Oreffo, Chief Scientific Officer of Renovos Biologics added:
“We are delighted our pioneering nanoclay product, RENOVITE® BMP-2, which provides unprecedented retention of therapeutics at the site of repair, has been granted Breakthrough Device Designation by the FDA. RENOVITE® BMP-2, the first product from our RENOVITE® platform technology, offers unprecedented opportunity for localised directed tissue regeneration with ease of use, safety and retention providing a transformative solution to address debilitating orthopaedic spinal conditions for an increasing aging population.”
“We are delighted our pioneering nanoclay product, RENOVITE® BMP-2, which provides unprecedented retention of therapeutics at the site of repair, has been granted Breakthrough Device Designation by the FDA. RENOVITE® BMP-2, the first product from our RENOVITE® platform technology, offers unprecedented opportunity for localised directed tissue regeneration with ease of use, safety and retention providing a transformative solution to address debilitating orthopaedic spinal conditions for an increasing aging population.”


